On September 14-15 and 18-20, 2566, regulatory officers of Food and Drug Administration (FDA) participated in a practical training on equipment and medical devices under the theme “Biocompatibility Testing of Medical Devices.” The training was organized by the ISO17025 Medical Device Biocompatibility Testing Laboratory in collaboration with the Biomedical Engineering Department, Faculty of Engineering, Mahidol University, and the Department of Industrial Economics, Ministry of Industry. The activity aimed to enhance the capabilities and human resources in the medical equipment and device industry. The training, focusing on equipment and medical devices, was conducted at no cost and had the objective of improving the proficiency and workforce in the medical equipment and device industry. Eight participants from the FDA attended the training.
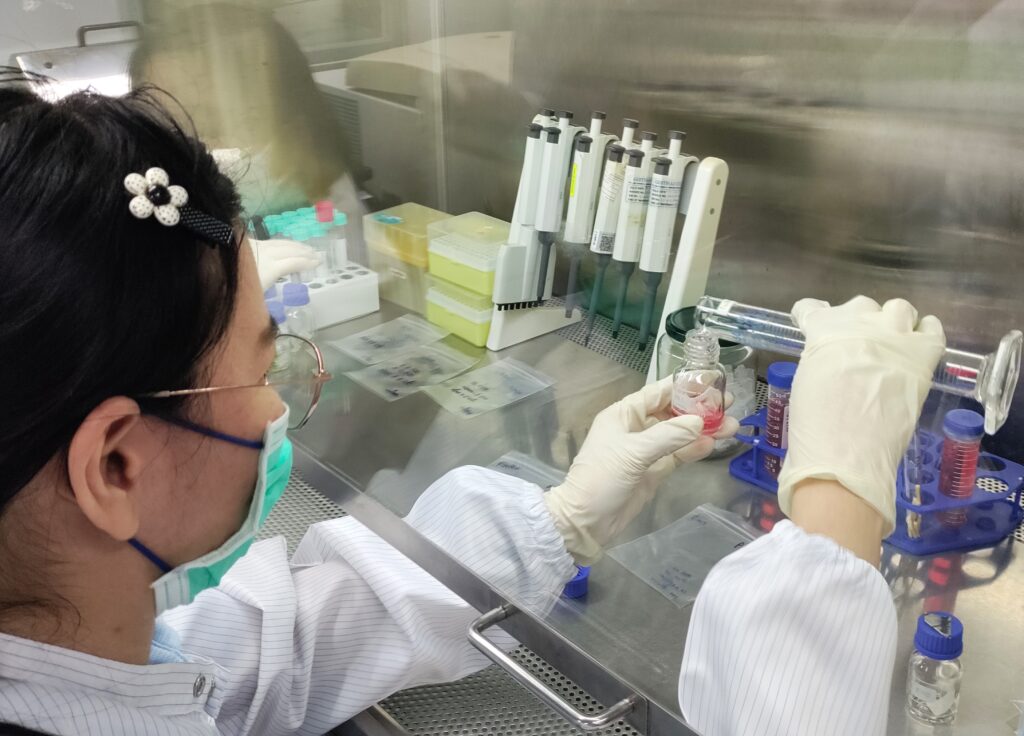
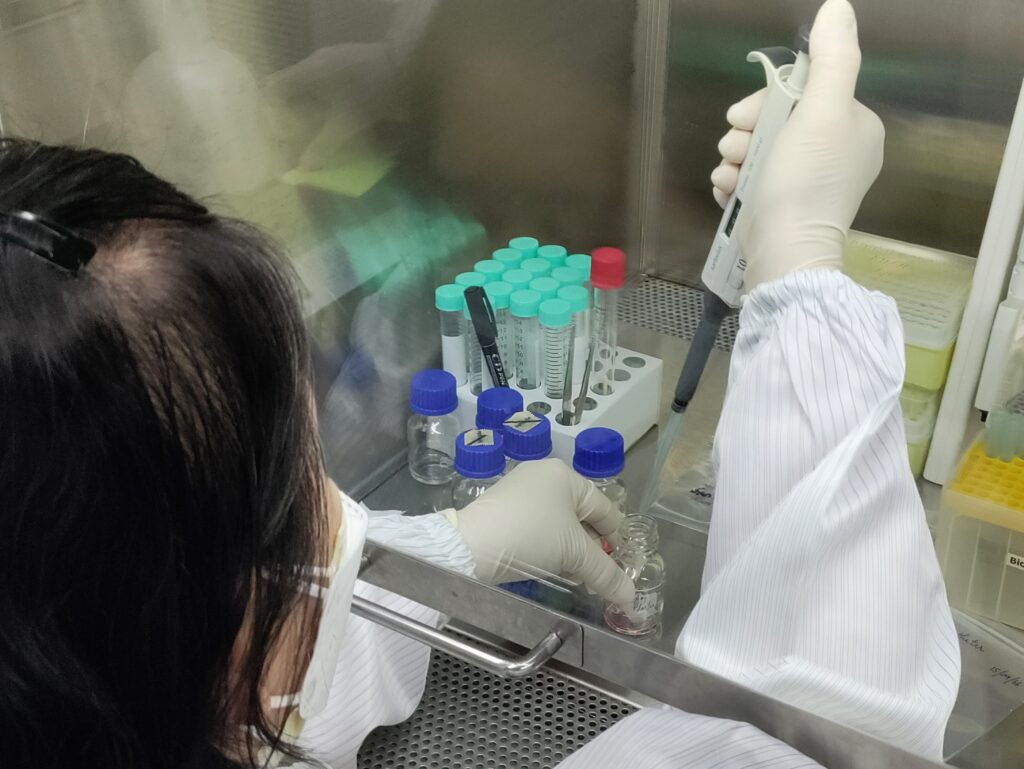
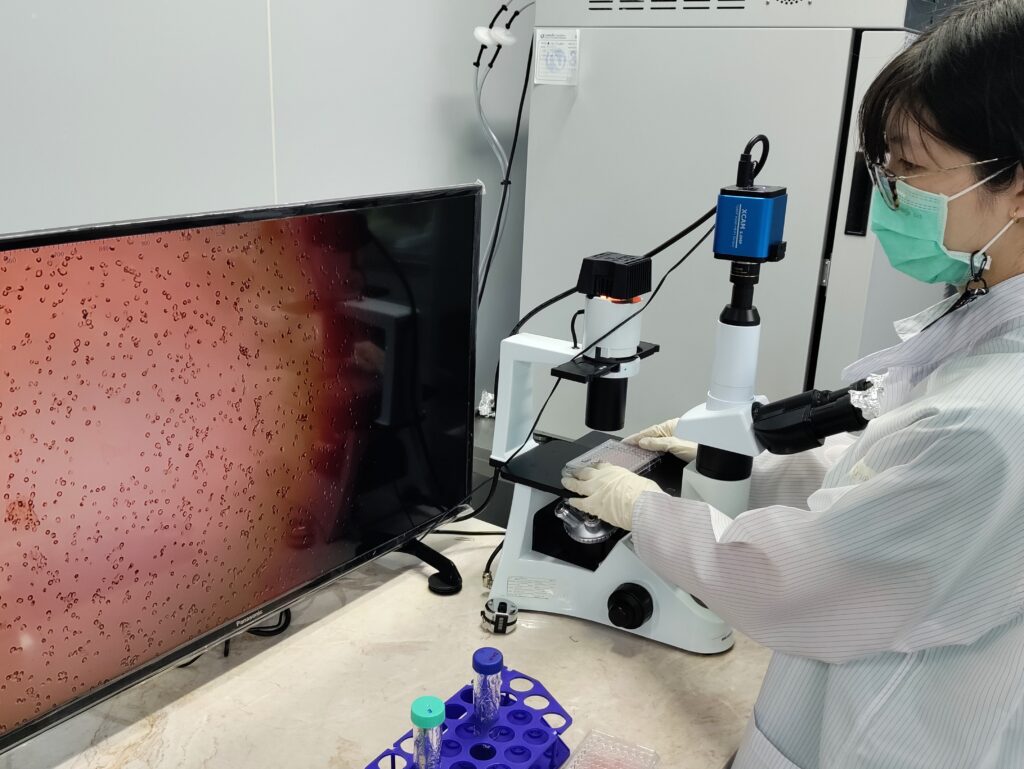
Microbial Culture Workshop
On July 17-18, 2024, The Laboratory for Biocompatibility Testing of Medical Device ISO17025 collaborated with the...



































































0 Comments