Laboratory for Biocompatibility Testing of Medical Devices
World-Class Biocompatibility Testing Laboratory
for Medical Device Industry
ISO/IEC 17025:2017 Certified
World-class standards for the competency of the laboratory, a qualification all professional laboratories require.
Timely and Responsive
In matters of business and saving patients, time is of the essence. Our Fast Track service offers results within 14 days.
Knowledge Builds Capability
Clear, concise and complete: our training services adheres to this principles to raise the capabilities of our attendees.
A Global Standard
For World-Class Service
The Laboratory for Biocompatibility Testing of Medical Devices is a certified ISO/IEC 17025:2017 laboratory that specializes in tests such as for biocompatibility and cytotoxicity, for medical devices such as gauze.
The laboratory operates under the Faculty of Engineering, Mahidol University, and is certified under global standards.
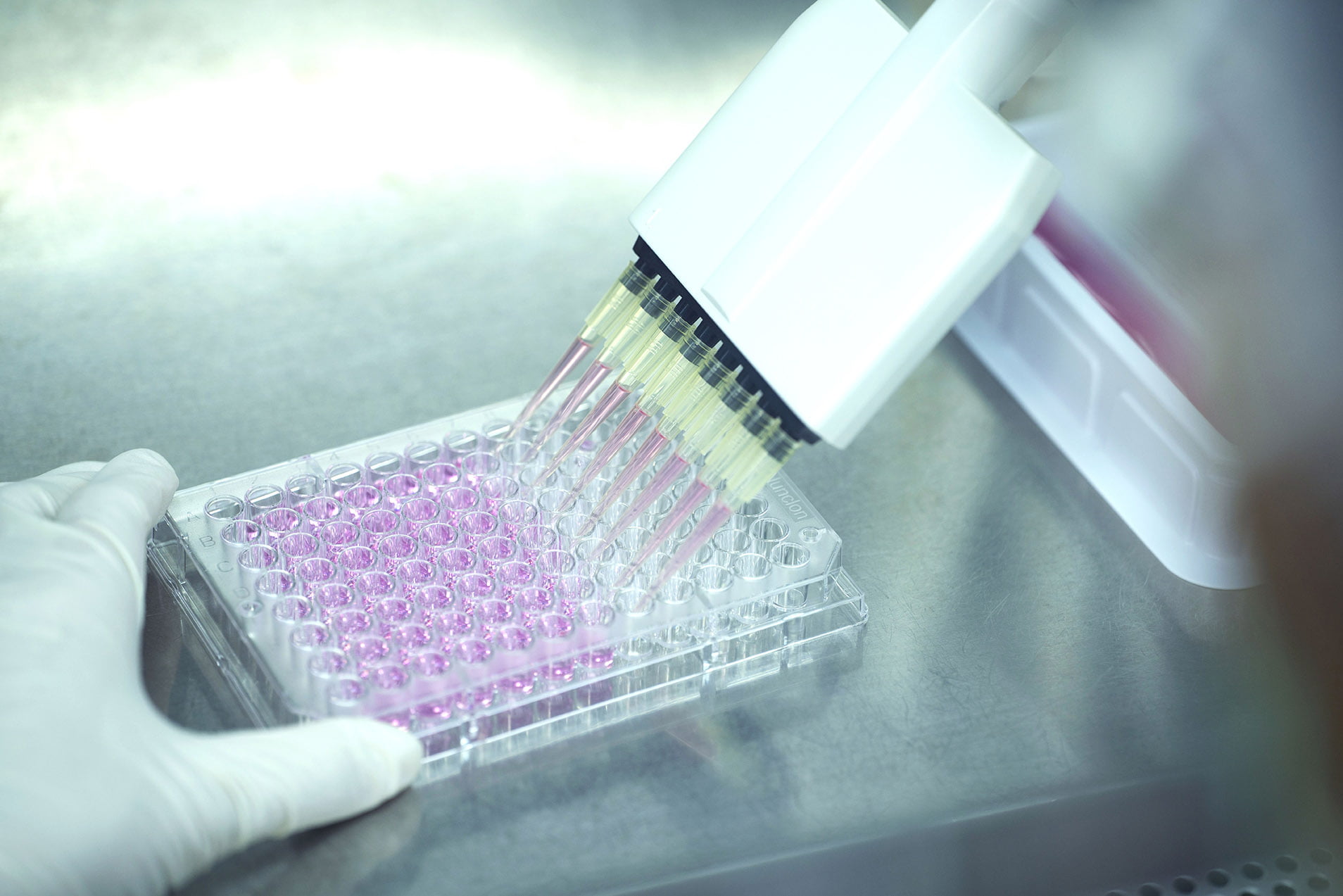
Testing Services for Medical Devices
Our services include cytotoxicity testing, hemolysis testing, microbiology testing, antibacterial susceptibility testing and composition analysis for medical devices.

Training Services for Industry Personnel
Our training modules for industry personnel and interested parties include topics such as working with cell cultures, biocompatibility testing of medical devices, and microbiology.
Our Latest News
Updates & Announcements
The Laboratory for Biocompatibility Testing of Medical Devices would like to present the results of our services, of which our customers were most satisfied with.
Laboratory visitation from Thailand Advanced Institute of Science and Technology and the Tokyo Institute of Technology (TAIST–Science Tokyo)
On 30 January 2026, the Laboratory for Biocompatibility Testing of Medical Devices, led by Associate Professor Dr. Norased Nasongkla, welcomed a delegation from TAIST–Science Tokyo, with the visit serving to strengthen international academic and research collaboration.
Laboratory visitation for Dr. Donald Balance and Sophia Wong from the University of Glasgow
On January 29, 2026, the Laboratory for Biocompatibility Testing of Medical Devices welcomed Dr. Donald Balance and Sophia Wong from the University of Glasgow for a visit to the Laboratory for Biocompatibility Testing of Medical Devices, Department of Biomedical Engineering, Faculty of Engineering, Mahidol University.


